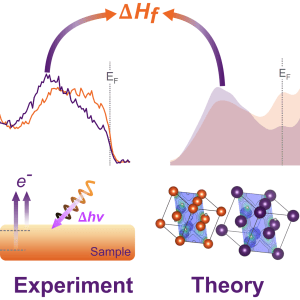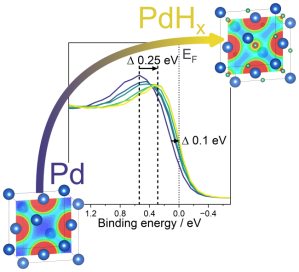
Palladium hydride is the model system for studying how hydrogen interacts with a metal host. Already in 1869, Thomas Graham, then Master of the Mint, published work showing that palladium was able to absorb large quantities of hydrogen. Yet, as with all hydrides, it has been difficult to directly probe its bulk electronic structure and chemical bonding.
Following from our work on yttrium and titanium metal hydride, we have now published work focusing on PdH. A key difference between Ti/Y hydride and Pd hydride is that Ti and Y form stable hydrides without external hydrogen pressure, whilst Pd does not, i.e. it needs active external hydrogen pressure to retain the hydrogen. Therefore, we had to change strategy switching from using ultra-high vacuum based hard X-ray photoelectron spectroscopy (HAXPES) to ambient-pressure HAXPES (AP-HAXPES). AP-HAXPES enabled us to measure the incorporation of hydrogen in Pd under 200 mbar of active hydrogen pressure at varying temperature. The use of hard X-rays not only provides improved probing depth into the solid, but allows the higher local hydrogen pressures necessary to observe the hydride formation. Combined with structural characterisation and density functional theory calculations we were able to explore the changes induced by the hydrogen incorporation in-situ and correlate this with the enthalpy of formation of the hydride.
The study was a team effort with friends and colleagues contributing their expertise. The HAXPES experiments for both published stories were conducted on beamline P22 at PETRA III (DESY), which has multiple end-stations enabling state-of-the-art studies supported by a fantastic local beamline team led by Dr Christoph Schlueter. Dr Lars Bannenberg and team contributed the samples and their extensive expertise in metal hydrides and their structural characterisation. Dr Laura Ratcliff was in charge of the theoretical efforts.